CURRENT RESEARCH
 Zebrafish ENS formation. From Baker et al., 2022 Zebrafish ENS formation. From Baker et al., 2022
|
Enteric Nervous System (ENS) Development and Differentiation
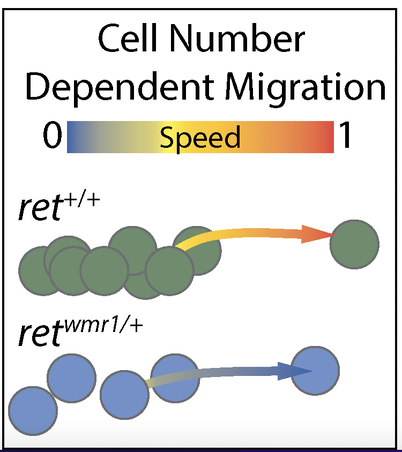
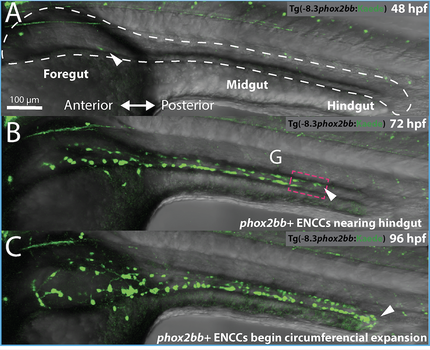
The ENS is a vast branch of the peripheral nervous system resident within the entire gut. It is also known as the gut brain and an essential portion of the gut-brain-axis. The main constituents of the ENS are hundreds of thousands of neurons and glia embedded within the walls of the gut. The ENS exhibits diverse enteric neuron subtypes and glial cells, which together regulates gut peristalsis, water balance and hormone secretions. While we know that the ENS is largely derived from the neural crest, we still know very little about its vast in vivo construction in the context of the gut. During zebrafish embryonic development, enteric neural crest cells migrate into the primitive gut as two migratory chains, surround the gut tube, and turn into neurons by the 5th day in development in order to form the ENS. Zebrafish offer a simplified vertebrate model to study ENS development. In our lab, we seek to understand how the ENS differentiates from the neural crest: We investigate how signaling factors, and other microenvironmental cues, interact with neural crest cells and gut tissues to orchestrate early ENS tissue patterning. We are also keen to understand the genetic underpinnings of enteric neuron diversification into specific neuron types. We leverage whole animal time-lapse live imaging and single cell tracking experiments to resolve the complex emergence of enteric neurons in the zebrafish gut, as shown on video left. Our recent study here https://doi.org/10.1242/dev.200668, in which we observed that enteric neural crest cells couple proliferation, migration speed, and cell density, to ensure proper gut tube colonization and timing of enteric neuron differentiation. |
 Neural crest development. From Howard and Uribe, 2022
Neural crest development. From Howard and Uribe, 2022
Neural Crest Cell Diversification
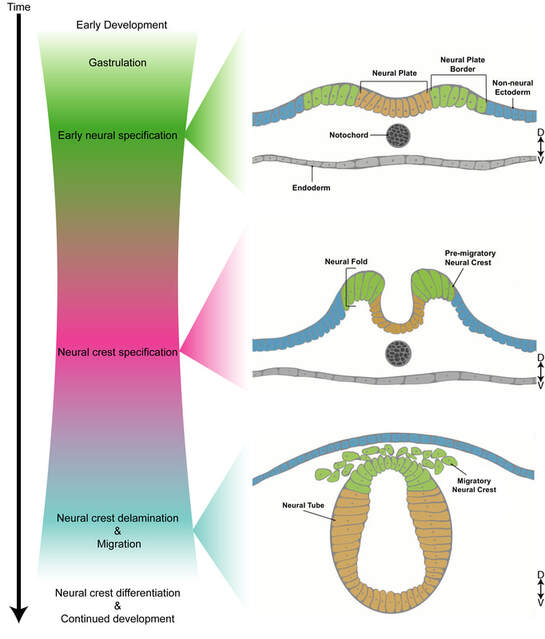
Neural crest cells are stem cells that migrate to various locations and give rise to diverse and fundamental cell types in the vertebrate body–including craniofacial tissues, cardiac cells and peripheral nervous system. Neural crest stem cells originate from the dorsal neural tube, a transient structure during development that will eventually give rise to the central nervous system. Some of the neural crest cells, called "vagal" neural crest cells, eventually can give rise to cells of the outflow tract of the heart, enteric peripheral ganglia, sympathetic ganglia, thymic connective tissue, as well as pigment cells of the skin.
What dictates whether neural crest will give rise to ganglia or other derivatives, such as pigment cells or connective tissues, remains elusive. To address this challenge, we study what regulates neural crest cell delineation, in the zebrafish embryo, a robust vertebrate model.
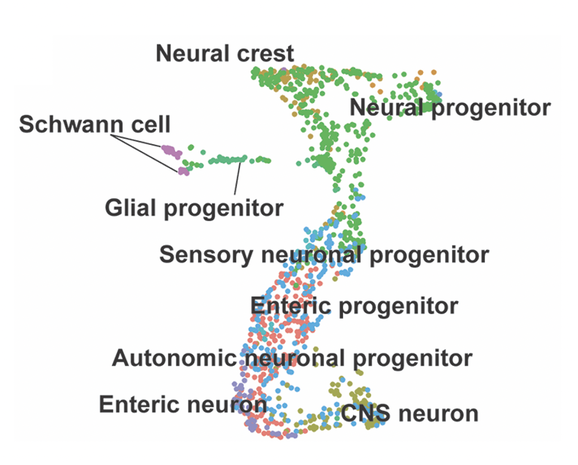
Towards that end, and begin shedding light on neural crest differentiation, we have undertaken studies on neural crest diversification in zebrafish using single-cell transcriptomics (Howard, Baker et al., 2021), and discovered dozens of transcriptionally-distinct neural crest-derived cellular subpopulations, greatly expanding the field's basic understanding of neural crest cell development. From these populations, we have uncovered previously unappreciated signatures of various genes, including those encoding transcription factors, implicating them in diversification of the vagal neural crest derivatives, including the ENS. Ongoing studies are building upon our recent single-cell discoveries in the lab right now.
Neural crest cells are stem cells that migrate to various locations and give rise to diverse and fundamental cell types in the vertebrate body–including craniofacial tissues, cardiac cells and peripheral nervous system. Neural crest stem cells originate from the dorsal neural tube, a transient structure during development that will eventually give rise to the central nervous system. Some of the neural crest cells, called "vagal" neural crest cells, eventually can give rise to cells of the outflow tract of the heart, enteric peripheral ganglia, sympathetic ganglia, thymic connective tissue, as well as pigment cells of the skin.
What dictates whether neural crest will give rise to ganglia or other derivatives, such as pigment cells or connective tissues, remains elusive. To address this challenge, we study what regulates neural crest cell delineation, in the zebrafish embryo, a robust vertebrate model.
Towards that end, and begin shedding light on neural crest differentiation, we have undertaken studies on neural crest diversification in zebrafish using single-cell transcriptomics (Howard, Baker et al., 2021), and discovered dozens of transcriptionally-distinct neural crest-derived cellular subpopulations, greatly expanding the field's basic understanding of neural crest cell development. From these populations, we have uncovered previously unappreciated signatures of various genes, including those encoding transcription factors, implicating them in diversification of the vagal neural crest derivatives, including the ENS. Ongoing studies are building upon our recent single-cell discoveries in the lab right now.
Relevance to Human Health and the Nervous System
Understanding the genetic programs and cellular interactions that drive stem cells to form the enteric nervous system is of crucial concern. From a large view, having knowledge about how neural crest differentiate into neural tissue is essential for understanding how cells make fundamental decisions in their native tissue context, and significantly, for also informing targeted designs for neural therapeutics.
Because improper neural crest development leads to developmental anomalies such as Hirschsprung disease (colonic aganglionosis), and neural crest-derived cancers, such as Melanoma and Neuroblastoma, there has been great interest in understanding the migration and differentiation of neural crest cells. It is important to study development of the ENS so that we can understand not only how it forms and functions, but also to help us to understand how things go wrong in various gastrointestinal autonomic neuropathies (Hirschsprung disease, Achalasia), as well as neural crest stem cell defects, such as when neural crest become cancerous.
Hirschsprung disease is characterized by a paucity of ganglia along variable lengths of the gut, with colonic aganglionosis being the most common form, occurring every 1 in 5000 births.. The current treatment for this pediatric developmental defect is surgical resection of the aganglionic intestinal segment--however eventual outcomes of patients varies greatly and most exhibit functional enteric defects throughout life--highlighting the need for alternative treatments and understanding the ontogeny of the disorder.
Melanoma and Neuroblastoma are neural crest-derived cancers affecting adults and children alike throughout the world. It is hypothesized that several genetic mutations in neural crest cell lineages are the basis for formation of neuroblastoma and melanoma cancers, however the signaling landscape conducive to formation of melanoma and neuroblastoma are not entirely known. Other current studies in the lab endeavor to understand how neural crest cell pathways may contribute to oncogenesis.
Understanding the genetic programs and cellular interactions that drive stem cells to form the enteric nervous system is of crucial concern. From a large view, having knowledge about how neural crest differentiate into neural tissue is essential for understanding how cells make fundamental decisions in their native tissue context, and significantly, for also informing targeted designs for neural therapeutics.
Because improper neural crest development leads to developmental anomalies such as Hirschsprung disease (colonic aganglionosis), and neural crest-derived cancers, such as Melanoma and Neuroblastoma, there has been great interest in understanding the migration and differentiation of neural crest cells. It is important to study development of the ENS so that we can understand not only how it forms and functions, but also to help us to understand how things go wrong in various gastrointestinal autonomic neuropathies (Hirschsprung disease, Achalasia), as well as neural crest stem cell defects, such as when neural crest become cancerous.
Hirschsprung disease is characterized by a paucity of ganglia along variable lengths of the gut, with colonic aganglionosis being the most common form, occurring every 1 in 5000 births.. The current treatment for this pediatric developmental defect is surgical resection of the aganglionic intestinal segment--however eventual outcomes of patients varies greatly and most exhibit functional enteric defects throughout life--highlighting the need for alternative treatments and understanding the ontogeny of the disorder.
Melanoma and Neuroblastoma are neural crest-derived cancers affecting adults and children alike throughout the world. It is hypothesized that several genetic mutations in neural crest cell lineages are the basis for formation of neuroblastoma and melanoma cancers, however the signaling landscape conducive to formation of melanoma and neuroblastoma are not entirely known. Other current studies in the lab endeavor to understand how neural crest cell pathways may contribute to oncogenesis.
Proudly powered by Weebly